NOx, short for nitrogen oxides, refers to a group of reactive gases that significantly contribute to air pollution. In this article, we delve into the definition of NOx, examine emissions based on fuel types, and explore effective methods to reduce NOx levels.

How NOx Forms and its Impact
Nitrogen oxide gases, NOx, are formed by NO and NO2. It contributes to air pollution and provides a major challenge regarding environmental and human health. They are the result of the burning of fossil fuels in many industrial operations, vehicle engines, and power stations.
Nitrogen oxide (NOx) emitted from different combustion processes is not retained in the atmosphere for an extended period. They will be converted to other compounds by chemical reactions, like NO combining with water and other materials. This is one of the causes of acid rain which is an undesirable result from an environmental perspective. Acid precipitation is hazardous to all water bodies and soils, thus adversely affecting plant life and the ecosystem at large.
Adverse Effects of High NOx Emissions
High levels of nitrogen oxide (NOx) emissions result in various derivatives, such as nitrogen dioxide, nitric acid, nitrous oxide, nitrates, and nitric oxide, each contributing to a spectrum of health and environmental impacts as reported by the EPA.
- Ground-level Ozone: NOx is the pseudo chemical formula for the Oxides of Nitrogen. The are 5 different oxides of nitrogen but the prevalent compounds in combustion are NO, NO2, and NO3. These three compounds react with heat and sunlight to produce ground-level ozone which is what we know as smog. This process leads to serious adverse effects, including damage to lung tissue, reduced lung function—especially in vulnerable populations like children, the elderly, and those with conditions such as asthma—and hazardous impacts on vegetation and crop yields.
- Particles: Nitric acid vapor and related particles form when NOx reacts with ammonia. These small particles have the potential to penetrate deeply into sensitive parts of the lungs, resulting in various human health concerns. Effects on breathing, damage to the respiratory system, and premature death may result after exposure to these particles.
- Global Warming: Nitrous oxide (NO2), a greenhouse gas, accumulates in the atmosphere alongside other greenhouse gases. This accumulation contributes to the Greenhouse Effect, causing a gradual rise in the Earth’s temperature. The long-term consequences include severe risks to human health, a rise in sea levels, and adverse changes to plant and animal habitats.
- Visibility Impairment: Higher levels of NOx in the air can block the transmission of light, leading to reduced visibility in urban areas. This impairment can have significant consequences for daily life and poses challenges for transportation and public safety.
- Toxic Chemicals: In the air, reactions of NOx with other organic chemicals result in the formation of various toxic products. These products may cause adverse biological effects, underscoring the importance of addressing and mitigating NOx emissions to safeguard public health.
- Water Quality Deterioration: Increased nitrogen loading in water bodies adversely affects the chemical balance of nutrients in aquatic life. This acceleration of “eutrophication” leads to oxygen depletion and a reduction in fish and shellfish populations. The overall deterioration of water quality has cascading effects on aquatic ecosystems.
- Acid Rain: NOx and sulfur dioxide in the air are major components of acids that fall to Earth as rain, fog, snow, or dry particles. Wind can carry these formations for long distances, resulting in acid rain. This phenomenon damages forests, causes deterioration of exposed substances, and renders water bodies acidic and unsuitable for life. Addressing NOx emissions is crucial to mitigate the far-reaching impact of acid rain on ecosystems.
Reviewing Emissions by Fuel Type
To understand how NOx emissions affect the environment, let’s look at different sources connected to different fuel types. Each kind of fuel contributes in its own way to the issues caused by nitrogen oxides.

- Mobile Sources – Automobiles and Other Vehicles: Approximately half of global NOx emissions stem from mobile sources, specifically automobiles and other mobile sources. The combustion engines in cars, trucks, buses, and airplanes release significant amounts of nitrogen oxides into the atmosphere.
- Stationary Sources – Electric Power Plants: Electric power plants, particularly those utilizing boilers, account for a substantial 40% of NOx emissions from stationary sources. The combustion of various fuels in power generation contributes significantly to the overall NOx levels.
- Anthropogenic Sources – Industrial Processes and Combustion Engines: The remaining 30% of NOx emissions result from diverse anthropogenic sources, including industrial boilers, incinerators, gas turbines, reciprocating spark ignition, and Diesel engines in stationary sources. Additionally, sectors such as iron and steel mills, cement manufacture, glass manufacture, petroleum refineries, and nitric acid manufacture contribute to this category.
- Biogenic, or Natural Sources: Beyond human activities, biogenic or natural sources play a role in NOx emissions. Lightning, forest fires, grass fires, trees, bushes, grasses, and yeasts release nitrogen oxides into the atmosphere.
Methods to Reduce NOX
Reducing NOx emissions from combustion sources involves employing proven strategies and technologies. Here are some effective approaches:
During Combustion
The following technologies reduce NOx during the combustion process:
- Wet Low Emissions (WLE) Technology
- Dry Low Emissions (WLE) Technology
- Catalytic Combustion
Low NOx technology in gas turbines centers around Wet Low Emissions (WLE) and Dry Low Emissions (DLE). The formation of NOx in a combustion process is dependent upon flame temperature. Therefore, reducing the flame temperature significantly reduces NOx formation. WLE and DLE technologies employ similar principles to reduce flame temperature, but their physical application is entirely different.
Wet Low Emissions (WLE)
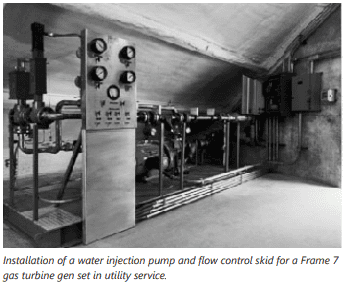
WLE is the older of the two technologies and is simply the injection of water into the gas turbine combustor. The water adds mass which absorbs heat (enthalpy) and reduces the flame temperature. WLE deploys on older gas turbines primarily because the combustor designs used for DLE cannot easily retrofitted onto the older gas turbines.
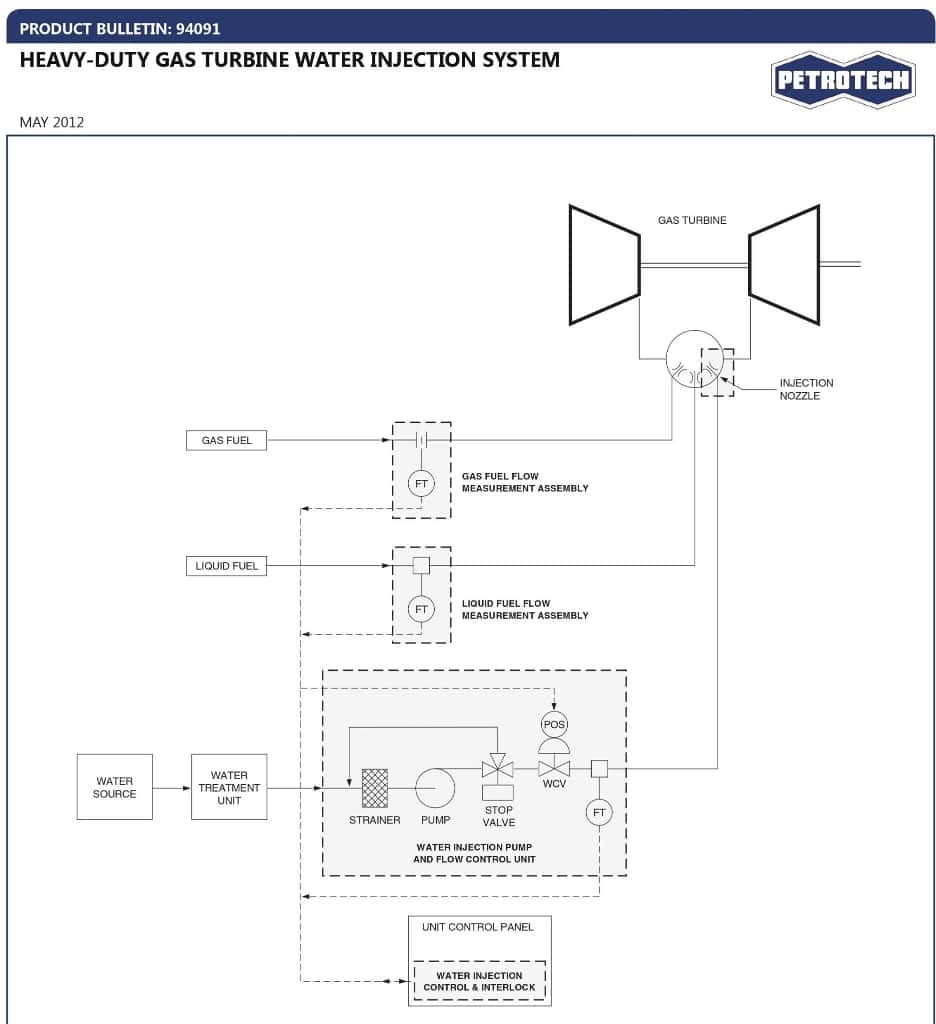
Petrotech builds and supplies water injection control systems as well as skid-mounted water injection pumping systems for gas turbine injection installations.
Dry Low Emissions (DLE)
DLE is the current best available technology for NOx gas turbine abatement. Here the original equipment manufacturers (OEMs) manufacture a specially designed combustor that uses excess air (also called lean pre-mixed combustion) rather than water to prevent the formation of NOx. The excess air absorbs heat (enthalpy) and reduces the flame temperature. The Turbine Fuel Regulation (TFR) controls for DLE also require more sophisticated algorithms than the legacy TFRs found on WLE installations. Petrotech can provide DLE TFR controls as well.
Catalytic combustion
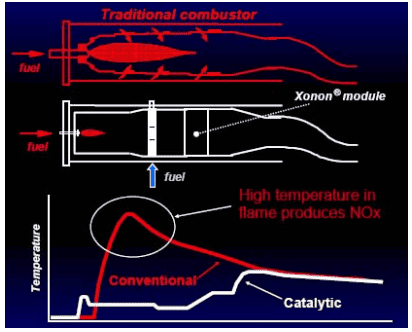
Catalytic combustion starts with the fuel getting injected upstream of the reactor, vaporizing and mixing with the inlet air. The fuel mixture then goes through the catalytic bed, where fuel and oxygen combustion occurs on the catalyst surface regardless of the fuel-air ratio. The heat of the exothermic reaction is released. The remaining fuel is combusted downstream at low temperatures to reduce the quantity of NOx formed.
Post-formation NOx-control technology
Selective Catalytic Reduction (SCR)
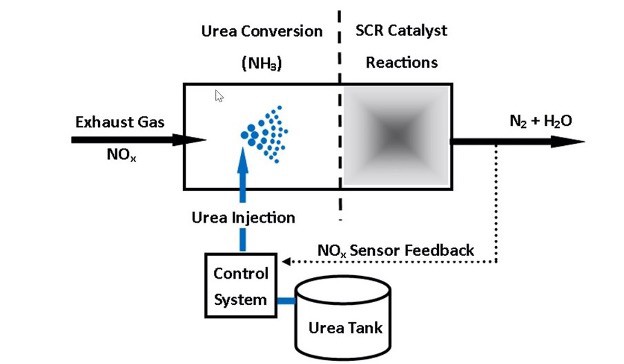
Selective Catalytic Reduction (SCR) stands as a post-formation NOx-control technology utilizing a catalyst and ammonia to reduce NOx to nitrogen and water. Here’s how it works:
- An ammonia/air or ammonia/steam mixture injects into the gas turbine exhaust gas stream.
- The gas, along with the injected mixture, passes through a catalyst where NOx is reduced.
To optimize the reaction, the temperature of the exhaust gas must fall within a specific range as it passes through the catalyst bed. Typically, SCR achieves removal efficiencies greater than 80%, irrespective of the combustion process or fuel type used.
While effective, SCR has certain drawbacks. It necessitates additional space for the catalyst and reactor vessel, as well as infrastructure for ammonia storage, distribution, and injection. Compliance with Federal Accidental Release Prevention rules may require the preparation and submission of a Risk Management Plan (RMP) for ammonia storage.
Precise control of ammonia injection is critical to SCR’s success. Inadequate ammonia amounts can result in unacceptably high NOx emission rates, while excess ammonia can lead to ammonia “slip,” where undesirable ammonia is vented to the atmosphere.
Selective Non-Catalytic Reduction (SNCR)
Selective non-catalytic NOx reduction involves injecting ammonia or urea into the flue gas to reduce NOx emissions. Success depends on the injection temperature, with 60% NOx removal achievable at the optimum temperature range.
Petrotech NOx Solutions
Petrotech specializes in NOx abatement solutions for gas turbines. The company’s expertise includes water injection control systems for Wet Low Emission (WLE) and sophisticated Turbine Fuel Regulation (TFR) controls for Dry Low Emission (DLE). Petrotech provides bespoke, innovative, and cost-effective solutions for NOx control in gas turbine applications. To get started on your project, request a quote with us today.
